Difference between revisions of "SUIT-011"
Beno Marija (talk | contribs) |
|||
| (40 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr= | |abbr=GM+S_OXPHOS+Rot_ET | ||
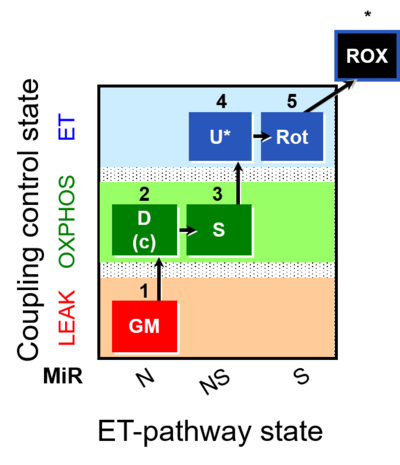
|description=[[File:1GM;2D;3S;4U;5Rot-.png| | |description=[[File:1GM;2D;3S;4U;5Rot-.png|400px|SUIT-011]] | ||
|info='''A | |info='''A: Maximum mitochondrial respiratory capacity ([[Oxidative phosphorylation|OXPHOS]] with [[NS-pathway control state|NS substrates]]) and coupling/pathway control''' | ||
}} | }} | ||
::: '''[[SUIT protocol pattern]]:''' 1GM;2D;2c;3S;4U;5Rot- | |||
| | |||
}} | The SUIT-011 protocols are designed to study physiologically relevant maximum mitochondrial respiratory capacity ([[Oxidative phosphorylation|OXPHOS]] with [[NS-pathway control state|NS substrates]]) and coupling/pathway control states. SUIT-011 gives information of the linear coupling control ([[LEAK respiration|''L'']]-[[Oxidative phosphorylation| ''P'']]) with NADH linked-substrates ([[GM-pathway control state|GM]]). GM and PM yield practically identical fluxes in human skeletal muscle fibres. However, PM is the superior alternative to GM, since the fractions of the N-pathway is lower and of the S-pathway is higher with GM compared to PM. PM, therefore, yields a more sensitive assay for the diagnosis of injuries in the N-linked pathway (compare [[SUIT-001]] and [[SUIT-004]]). Moreover, SUIT-011 allows the evaluation of the coupling-control state ([[Oxidative phosphorylation|''P'']]-[[ET capacity| ''E'']]) with NADH and succinate linked-substrates ([[NS-pathway control state|NS]]) and the pathway control in OXPHOS ([[NS-pathway control state|NS]]) and ET state ([[NS-pathway control state|NS]] and [[Succinate pathway control state|S]]). SUIT-011 can be extended with the CIV assay module. | ||
::: '''[[ | |||
::: '''[[SUIT protocol | |||
__TOC__ | |||
Communicated by [[Doerrier C]] and [[Gnaiger E]] (last update 2019-06-05) | |||
== Specific SUIT protocols == | |||
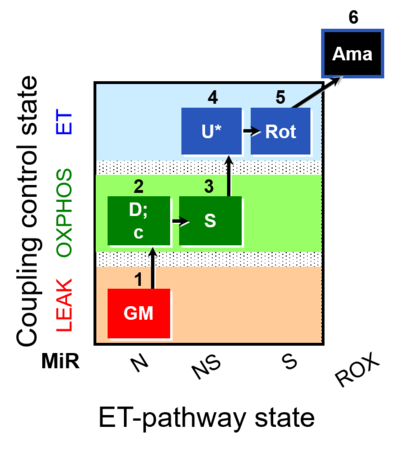
[[File:1GM;2D;2c;3S;4U;5Rot;6Ama.png|400px]] | |||
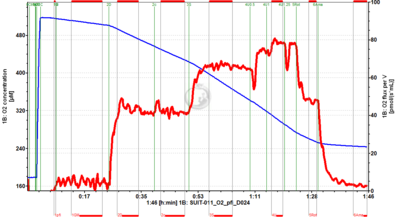
[[File:D024_O2_traces.png|400px]] | |||
* [[SUIT-011 O2 pfi D024]] for permeabilized fibers | |||
{{Template:SUIT-011}} | |||
== Strengths and limitations == | |||
:::* Comparison of GM- with PM-capacity yields important information on N-pathway respiratory control upstream of CI ([[Lemieux 2017 Sci Rep|Lemieux ''et al.'' 2017]]; [[Votion 2012 PLoS One|Votion ''et al.'' 2012]]). | |||
:::* A succinate concentration of >10 mM may be required for saturating SE capacity. | |||
:::* Rox might be inhibited slightly further by inhibition of CIV by cyanide (KCN; 1 μM). But cyanide inhibits not only CIV, but also catalase and other oxygenases involved in ROX. | |||
:::+ NS-OXPHOS capacity provides a physiologically relevant estimate of maximum mitochondrial respiratory capacity. | |||
:::+ Glutamate is easier to prepare compared to pyruvate. | |||
:::+ Application of the cytochrome ''c'' test early in the protocol ensures comparability of all states in case of any effect of ''c''. | |||
:::+ Reasonable duration of the experiment. | |||
:::- GM and PM yield typically identical fluxes in human skeletal muscle fibres. However, PM is the superior alternative to GM: the fraction of the N-pathway is lower and of the S-pathway is higher with GM compared to PM (GM<sub>''P''</sub> is inhibited by the CII inhibitor malonic acid to a larger extent than PM<sub>''P''</sub>). PM, therefore, yields a more sensitive assay for the diagnosis of injuries in the N-pathway, since an impairment of N-pathway capacity can be compensated partially by activation of the S-pathway. This is a disadvantage compared to SUIT-004 and SUIT-008 for diagnosis of N-capacity. | |||
:::- To detect an additive effect of P after GM<sub>''P''</sub>, pyruvate would have to be added as step 3 (before S). However, inhibition of respiration was observed after titration of P (5 mM) in horse skeletal muscle fibres (Votion et al 2012), which was not the case when P was titrated in steps of 1 mM. | |||
:::- When evaluating the additive effect of the N- and S-pathway, it has to be considered that NS<sub>''P''</sub>- and NS<sub>''E''</sub>-capacities can only be compared with N<sub>''P''</sub>- and S<sub>''E''</sub>-capacities. This is not a problem when NS<sub>''P''</sub> = NS<sub>''E''</sub> (Gnaiger 2009). Otherwise, it may be assumed that S<sub>''P''</sub> = S<sub>''E''</sub> (Votion et al 2012), such that NS<sub>''P''</sub> can be compared with N<sub>''P''</sub> + S<sub>''P''</sub>. SUIT-004 should be chosen for the additive effect in the ET-state. | |||
:::- ''Rox'' may be lower in substrate states earlier in the SUIT protocol. Therefore, this ''Rox'' measurement is frequently taken as a methodological control rather than as the final basis of ''Rox'' correction of mitochondrial respiration (mt). | |||
:::- Careful washing is required after the experiment to avoid carry-over of inhibitors and uncoupler. | |||
:::- CIV activity is not measured, to save experimental time. | |||
== Compare SUIT protocols == | |||
::::* GM and PM yield typically identical fluxes in human skeletal muscle fibres. | |||
::::* [[SUIT-004]] 1PM;2D;3U;4S;5Rot-: SUIT-004 allows the evaluation of the linear coupling control ([[LEAK respiration|''L'']]-[[Oxidative phosphorylation| ''P'']]) with PM (instead of GM) as NADH linked-substrates. Moreover, in SUIT-004 the linear coupling control from [[Oxidative phosphorylation| ''P'']] to [[ET capacity| ''E'']] (with PM) and the ET-pathway state in[[NS-pathway control state| NS-]] and [[Succinate pathway control state| S-pathways]] can be assessed. | |||
::::* [[SUIT-008]] 1PM;2D;3G;4S;5U;6Rot-: SUIT-008 protocols are designed to assess the additivity between the [[NADH_Electron_transfer-pathway_state| N-]] and [[Succinate pathway control state| S-pathway]] in the [[Q-junction]], providing a physiologically relevant estimate of maximum mitochondrial respiratory capacity. | |||
::::* [[SUIT-001]] 1PM;2D;3U;4G;5S;6Oct;7Rot;8Gp-: [[SUIT reference protocol]] 1 (RP1)gives information of the linear coupling control ([[LEAK respiration|''L'']]-[[Oxidative phosphorylation| ''P'']]-[[ET capacity| ''E'']]) with NADH linked-substrates ([[PM-pathway control state|PM]]). Moreover, the pathway control in ET state ([[NADH_Electron_transfer-pathway_state|N]], [[NS-pathway control state| NS]], [[FNS]], [[Succinate pathway control state| S]] and [[SGp-pathway control state| SGp]] pathways) can be evaluated by using this SUIT protocol. | |||
::::* [[SUIT-028]]: PGM as NADH linked-substrates. | |||
== References == | == References == | ||
{{#ask:[[Category:Publications]] [[Additional label::SUIT-011]] | {{#ask:[[Category:Publications]] [[Instrument and method::O2k-Protocol]] [[Additional label::SUIT-011]] | ||
|?Was published in year=Year | |?Was published in year=Year | ||
|?Has title=Reference | |?Has title=Reference | ||
| Line 22: | Line 57: | ||
}} | }} | ||
{{MitoPedia concepts | |||
|mitopedia concept=MiP concept, SUIT protocol, Recommended | |||
}} | |||
{{MitoPedia methods | |||
|mitopedia method=Respirometry | |||
{ | }} | ||
| | |||
= | |||
Latest revision as of 15:35, 8 June 2020
Description
Abbreviation: GM+S_OXPHOS+Rot_ET
Reference: A: Maximum mitochondrial respiratory capacity (OXPHOS with NS substrates) and coupling/pathway control
- SUIT protocol pattern: 1GM;2D;2c;3S;4U;5Rot-
The SUIT-011 protocols are designed to study physiologically relevant maximum mitochondrial respiratory capacity (OXPHOS with NS substrates) and coupling/pathway control states. SUIT-011 gives information of the linear coupling control (L- P) with NADH linked-substrates (GM). GM and PM yield practically identical fluxes in human skeletal muscle fibres. However, PM is the superior alternative to GM, since the fractions of the N-pathway is lower and of the S-pathway is higher with GM compared to PM. PM, therefore, yields a more sensitive assay for the diagnosis of injuries in the N-linked pathway (compare SUIT-001 and SUIT-004). Moreover, SUIT-011 allows the evaluation of the coupling-control state (P- E) with NADH and succinate linked-substrates (NS) and the pathway control in OXPHOS (NS) and ET state (NS and S). SUIT-011 can be extended with the CIV assay module.
Communicated by Doerrier C and Gnaiger E (last update 2019-06-05)
Specific SUIT protocols
- SUIT-011 O2 pfi D024 for permeabilized fibers
Steps and respiratory states
| Step | State | Pathway | Q-junction | Comment - Events (E) and Marks (M) |
|---|---|---|---|---|
| 1GM | GML(n) | N | CI | 1GM
|
| 2D | GMP | N | CI | 1GM;2D
|
| 2c | GMcP | N | CI | 1GM;2D;2c
|
| 3S | GMSP | NS | CI&II | 1GM;2D;2c;3S
|
| 4U | GMSE | NS | CI&II | 1GM;2D;2c;3S;4U
|
| 5Rot | SE | S | CII | 1GM;2D;2c;3S;4U;5Rot
|
| 6Ama | ROX | 1GM;2D;2c;3S;4U;5Rot;6Ama
|
| Step | Respiratory state | Pathway control | ET-Complex | Comment |
|---|---|---|---|---|
| ## AsTm | AsTmE | CIV | CIV | |
| ## Azd | CHB |
- Bioblast links: SUIT protocols - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coupling control
- Pathway control
- Main fuel substrates
- » Glutamate, G
- » Glycerophosphate, Gp
- » Malate, M
- » Octanoylcarnitine, Oct
- » Pyruvate, P
- » Succinate, S
- Main fuel substrates
- Glossary
Strengths and limitations
- Comparison of GM- with PM-capacity yields important information on N-pathway respiratory control upstream of CI (Lemieux et al. 2017; Votion et al. 2012).
- A succinate concentration of >10 mM may be required for saturating SE capacity.
- Rox might be inhibited slightly further by inhibition of CIV by cyanide (KCN; 1 μM). But cyanide inhibits not only CIV, but also catalase and other oxygenases involved in ROX.
- + NS-OXPHOS capacity provides a physiologically relevant estimate of maximum mitochondrial respiratory capacity.
- + Glutamate is easier to prepare compared to pyruvate.
- + Application of the cytochrome c test early in the protocol ensures comparability of all states in case of any effect of c.
- + Reasonable duration of the experiment.
- - GM and PM yield typically identical fluxes in human skeletal muscle fibres. However, PM is the superior alternative to GM: the fraction of the N-pathway is lower and of the S-pathway is higher with GM compared to PM (GMP is inhibited by the CII inhibitor malonic acid to a larger extent than PMP). PM, therefore, yields a more sensitive assay for the diagnosis of injuries in the N-pathway, since an impairment of N-pathway capacity can be compensated partially by activation of the S-pathway. This is a disadvantage compared to SUIT-004 and SUIT-008 for diagnosis of N-capacity.
- - To detect an additive effect of P after GMP, pyruvate would have to be added as step 3 (before S). However, inhibition of respiration was observed after titration of P (5 mM) in horse skeletal muscle fibres (Votion et al 2012), which was not the case when P was titrated in steps of 1 mM.
- - When evaluating the additive effect of the N- and S-pathway, it has to be considered that NSP- and NSE-capacities can only be compared with NP- and SE-capacities. This is not a problem when NSP = NSE (Gnaiger 2009). Otherwise, it may be assumed that SP = SE (Votion et al 2012), such that NSP can be compared with NP + SP. SUIT-004 should be chosen for the additive effect in the ET-state.
- - Rox may be lower in substrate states earlier in the SUIT protocol. Therefore, this Rox measurement is frequently taken as a methodological control rather than as the final basis of Rox correction of mitochondrial respiration (mt).
- - Careful washing is required after the experiment to avoid carry-over of inhibitors and uncoupler.
- - CIV activity is not measured, to save experimental time.
Compare SUIT protocols
- GM and PM yield typically identical fluxes in human skeletal muscle fibres.
- SUIT-004 1PM;2D;3U;4S;5Rot-: SUIT-004 allows the evaluation of the linear coupling control (L- P) with PM (instead of GM) as NADH linked-substrates. Moreover, in SUIT-004 the linear coupling control from P to E (with PM) and the ET-pathway state in NS- and S-pathways can be assessed.
- SUIT-008 1PM;2D;3G;4S;5U;6Rot-: SUIT-008 protocols are designed to assess the additivity between the N- and S-pathway in the Q-junction, providing a physiologically relevant estimate of maximum mitochondrial respiratory capacity.
- SUIT-001 1PM;2D;3U;4G;5S;6Oct;7Rot;8Gp-: SUIT reference protocol 1 (RP1)gives information of the linear coupling control (L- P- E) with NADH linked-substrates (PM). Moreover, the pathway control in ET state (N, NS, FNS, S and SGp pathways) can be evaluated by using this SUIT protocol.
- SUIT-028: PGM as NADH linked-substrates.
References
MitoPedia concepts: MiP concept, SUIT protocol, Recommended
MitoPedia methods:
Respirometry