Difference between revisions of "Malate"
| Line 3: | Line 3: | ||
|description=[[File:Malic_acid.jpg|left|100px|Malic acid]] | |description=[[File:Malic_acid.jpg|left|100px|Malic acid]] | ||
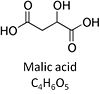
'''Malic acid''', C<sub>4</sub>H<sub>6</sub>O<sub>5</sub>, occurs under physiological conditions as the anion '''malate<sup>2-</sup>, M''', with p''K''<sub>a1</sub> = 3.40 and p''K''<sub>a2</sub> = 5.20. L-Malate is formed from [[fumarate]] in the [[TCA cycle]] in the mitochondrial matrix, where it is the substrate of [[malate dehydrogenase]] oxidized to [[oxaloacetate]]. Malate is also formed in the cytosol. It cannot permeate through the lipid bilayer of membranes and hence requires a carrier ([[dicarboxylate carrier]], [[tricarboxylate carrier]] and [[2-oxoglutarate carrier]]). Malate alone cannot support respiration of [[Mitochondrial preparations|mt-preparations]] from most tissues, since oxaloacetate accumulates in the absence of [[pyruvate]] or [[glutamate]]. | '''Malic acid''', C<sub>4</sub>H<sub>6</sub>O<sub>5</sub>, occurs under physiological conditions as the anion '''malate<sup>2-</sup>, M''', with p''K''<sub>a1</sub> = 3.40 and p''K''<sub>a2</sub> = 5.20. L-Malate is formed from [[fumarate]] in the [[TCA cycle]] in the mitochondrial matrix, where it is the substrate of [[malate dehydrogenase]] oxidized to [[oxaloacetate]]. Malate is also formed in the cytosol. It cannot permeate through the lipid bilayer of membranes and hence requires a carrier ([[dicarboxylate carrier]], [[tricarboxylate carrier]] and [[2-oxoglutarate carrier]]). Malate alone cannot support respiration of [[Mitochondrial preparations|mt-preparations]] from most tissues, since oxaloacetate accumulates in the absence of [[pyruvate]] or [[glutamate]]. | ||
|info=[ | |info=[[Gnaiger 2014 MitoPathways]] | ||
|type=Respiration | |type=Respiration | ||
}} | }} | ||
| Line 32: | Line 32: | ||
::* Final concenteration: 0.5 (2.0) mM. | ::* Final concenteration: 0.5 (2.0) mM. | ||
[[Image:BB-Bioblast.jpg|left|30px|link= | [[Image:BB-Bioblast.jpg|left|30px|link=Bioblast:About|Bioblast wiki]] | ||
== Malate: 0.5 mM versus 2 mM in HRR == | == Malate: 0.5 mM versus 2 mM in HRR == | ||
In mitochondrial preparations obtained from a diversity of tissues and organisms malate at concentrations >0.5 mM exerted an inhibitory effect on CII-linked and CI&II-linked respiration, whereas 0.5 mM malate was saturating for CI linked respiration. Selection of an optimum malate concentration for SUIT protocols is a compromise. Current investigations point towards 2 mM malate, but a final result is not yet available. | In mitochondrial preparations obtained from a diversity of tissues and organisms malate at concentrations >0.5 mM exerted an inhibitory effect on CII-linked and CI&II-linked respiration, whereas 0.5 mM malate was saturating for CI linked respiration. Selection of an optimum malate concentration for SUIT protocols is a compromise. Current investigations point towards 2 mM malate, but a final result is not yet available. | ||
* ''Further details'': »[[Talk:Malate|Discussion]]. | * ''Further details'': »[[Talk:Malate|Discussion]]. | ||
Revision as of 19:25, 2 March 2015
Description
Malic acid, C4H6O5, occurs under physiological conditions as the anion malate2-, M, with pKa1 = 3.40 and pKa2 = 5.20. L-Malate is formed from fumarate in the TCA cycle in the mitochondrial matrix, where it is the substrate of malate dehydrogenase oxidized to oxaloacetate. Malate is also formed in the cytosol. It cannot permeate through the lipid bilayer of membranes and hence requires a carrier (dicarboxylate carrier, tricarboxylate carrier and 2-oxoglutarate carrier). Malate alone cannot support respiration of mt-preparations from most tissues, since oxaloacetate accumulates in the absence of pyruvate or glutamate.
Abbreviation: M
Reference: Gnaiger 2014 MitoPathways
MitoPedia topics: Substrate and metabolite
Application in HRR
M: Malate (L-Malic acid, C4H605); Sigma M 1000, 100 g, store at RT; FW = 134.1.
Preparation of 400 (800) mM stock solution (dissolved in H2O):
- 1) Weigh 268.2 (536.4) mg of L-malic acid.
- 2) Add 3 ml H2O.
- 3) Neutralize with 10 N KOH (approx. 350 µl).
- 4) Adjust final volume to 5 ml (in 5 ml volumetric glass flask).
- 5) Divide into 0.5 ml portions.
- 6) Store frozen at -20 °C.
Comment: 800 mM stock until 2013-11-20.
Oxygraph-2k manual titrations >> MiPNet09.12 O2k-Titrations
- Titration volume: 2.5 µl 400 mM (5 µl 800 mM) using a 25 µl Hamilton syringe (2 ml O2k-chamber).
- Final concenteration: 0.5 (2.0) mM.
Malate: 0.5 mM versus 2 mM in HRR
In mitochondrial preparations obtained from a diversity of tissues and organisms malate at concentrations >0.5 mM exerted an inhibitory effect on CII-linked and CI&II-linked respiration, whereas 0.5 mM malate was saturating for CI linked respiration. Selection of an optimum malate concentration for SUIT protocols is a compromise. Current investigations point towards 2 mM malate, but a final result is not yet available.
- Further details: »Discussion.