Difference between revisions of "MitoFit-QCS"
From Bioblast
Laner Verena (talk | contribs) |
|||
| (13 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Oroboros highlights page name}} | ||
<br /> | |||
:::: <big><big>The project MitoFit highlights the benefits of mitochondrial fitness.</big></big> | :::: <big><big>The project MitoFit highlights the benefits of mitochondrial fitness.</big></big> | ||
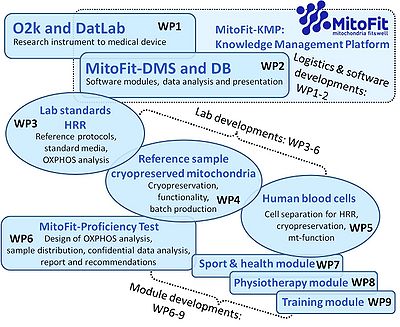
[[File:MitoFit Work packages.jpg|right|400px|link=K-Regio MitoFit#Workpackages|MitoFit Workpackages]] | |||
<br /> | |||
<big><big><big>'''WP3'''</big></big></big> | |||
:::: <big><big><big>'''Laboratory standards for high-resolution respirometry: towards a QCS'''</big></big></big> | |||
__TOC__ | |||
== Abstract == | |||
:::: The aim is to develop and establish standard experimental procedures, standard protocols and a library of substrate-uncoupler-inhibitor titration (SUIT) protocols to support applications of high-resolution respirometry and O2k-Fluorometry in basic research and diagnostic assays. These represent essential developments towards a new quality control system (QCS). Current studies of mitochondrial respiratory function are characterized by an apparently arbitrary diversity of experimental procedures, lack of standardized protocols and consequently the challenge for any project to select or systematically evaluate an appropriate assay protocol addressing a specific problem (Gnaiger 2014). To fill this gap, presently available protocols will be systematically listed, their strength and limitations tested and discussed, and finally a critically selected set of standard procedures and protocols will be developed and documented in a library of protocols. This library is intended to assist in applications of the O2k-technology for the diagnosis of mitochondrial function in cells and tissues. Present limitations of inadequate approaches (Rogers et al 2011) and outdated concepts (Barrientos et al 2009) have to be overcome, providing a rationale for establishing a set of basic reference protocols. | :::: The aim is to develop and establish standard experimental procedures, standard protocols and a library of substrate-uncoupler-inhibitor titration (SUIT) protocols to support applications of high-resolution respirometry and O2k-Fluorometry in basic research and diagnostic assays. These represent essential developments towards a new quality control system (QCS). Current studies of mitochondrial respiratory function are characterized by an apparently arbitrary diversity of experimental procedures, lack of standardized protocols and consequently the challenge for any project to select or systematically evaluate an appropriate assay protocol addressing a specific problem (Gnaiger 2014). To fill this gap, presently available protocols will be systematically listed, their strength and limitations tested and discussed, and finally a critically selected set of standard procedures and protocols will be developed and documented in a library of protocols. This library is intended to assist in applications of the O2k-technology for the diagnosis of mitochondrial function in cells and tissues. Present limitations of inadequate approaches (Rogers et al 2011) and outdated concepts (Barrientos et al 2009) have to be overcome, providing a rationale for establishing a set of basic reference protocols. | ||
::::* [[ISO 15189: | == Progress and next steps == | ||
::::» [[Sumbalova 2015 Abstract MiP2015]]: High-resolution measurement of mitochondrial membrane potential and respiration – comparison of potentiometric and fluorometric methods. | |||
::::» [[MitoFit Science Camp 2016 Kuehtai AT]] | |||
:::::* [[Sumbalova 2016a Abstract MitoFit Science Camp 2016]]: Optimizing strategies on the malate concentration in SUIT protocols. | |||
:::::* [[Krumschnabel 2016a Abstract MitoFit Science Camp 2016]]: O2k-Protocols: Mitochondrial preparations for HRR. | |||
:::::* [[Krumschnabel 2016b Abstract MitoFit Science Camp 2016]]: O2k-MultiSensor: Mitochondrial respiration media for HRR and simultaneous O2k-Fluorometry. | |||
:::::* [[Neufer 2016 Abstract MitoFit Science Camp 2016]]: Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. | |||
:::::* [[Bezuidenhout 2016a Abstract MitoFit Science Camp 2016]]: Comparison of oxygen dependence of respiration in permeabilized mouse skeletal muscle fibers in two respiration media, MiR06Cr and Buffer Z containing Ctl, Cr and Blebbistatin. | |||
:::::* [[Kane 2016 Abstract MitoFit Science Camp 2016]]: Effects of inhibiting myosin-ATPase on mitochondrial respiratory capacity in permeabilized skeletal muscle. | |||
:::::* [[Garcia-Roves 2016b Abstract MitoFit Science Camp 2016]]: Technical perspective of high-resolution respirometry in permeabilized skinned muscle fibers from different mouse models. | |||
::::» [[MitoFit Quality Control System]] | |||
::::» [[MitoPedia: SUIT]] | |||
== Links and references == | |||
::::» [[ISO 15189:2012 Medical laboratories — Particular requirements for quality and competence]] | |||
::::» [[ISO 9001:2015 Quality management systems - requirements]] | |||
:::: | ::::» [[MitoEAGLE| COST MitoEAGLE project]] | ||
:::: | ::::« [[K-Regio MitoFit#Workpackages|Back to overview]] | ||
Latest revision as of 13:26, 3 April 2022
MitoFit-QCS
- The project MitoFit highlights the benefits of mitochondrial fitness.
WP3
- Laboratory standards for high-resolution respirometry: towards a QCS
Abstract
- The aim is to develop and establish standard experimental procedures, standard protocols and a library of substrate-uncoupler-inhibitor titration (SUIT) protocols to support applications of high-resolution respirometry and O2k-Fluorometry in basic research and diagnostic assays. These represent essential developments towards a new quality control system (QCS). Current studies of mitochondrial respiratory function are characterized by an apparently arbitrary diversity of experimental procedures, lack of standardized protocols and consequently the challenge for any project to select or systematically evaluate an appropriate assay protocol addressing a specific problem (Gnaiger 2014). To fill this gap, presently available protocols will be systematically listed, their strength and limitations tested and discussed, and finally a critically selected set of standard procedures and protocols will be developed and documented in a library of protocols. This library is intended to assist in applications of the O2k-technology for the diagnosis of mitochondrial function in cells and tissues. Present limitations of inadequate approaches (Rogers et al 2011) and outdated concepts (Barrientos et al 2009) have to be overcome, providing a rationale for establishing a set of basic reference protocols.
Progress and next steps
- » Sumbalova 2015 Abstract MiP2015: High-resolution measurement of mitochondrial membrane potential and respiration – comparison of potentiometric and fluorometric methods.
- » MitoFit Science Camp 2016 Kuehtai AT
- Sumbalova 2016a Abstract MitoFit Science Camp 2016: Optimizing strategies on the malate concentration in SUIT protocols.
- Krumschnabel 2016a Abstract MitoFit Science Camp 2016: O2k-Protocols: Mitochondrial preparations for HRR.
- Krumschnabel 2016b Abstract MitoFit Science Camp 2016: O2k-MultiSensor: Mitochondrial respiration media for HRR and simultaneous O2k-Fluorometry.
- Neufer 2016 Abstract MitoFit Science Camp 2016: Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers.
- Bezuidenhout 2016a Abstract MitoFit Science Camp 2016: Comparison of oxygen dependence of respiration in permeabilized mouse skeletal muscle fibers in two respiration media, MiR06Cr and Buffer Z containing Ctl, Cr and Blebbistatin.
- Kane 2016 Abstract MitoFit Science Camp 2016: Effects of inhibiting myosin-ATPase on mitochondrial respiratory capacity in permeabilized skeletal muscle.
- Garcia-Roves 2016b Abstract MitoFit Science Camp 2016: Technical perspective of high-resolution respirometry in permeabilized skinned muscle fibers from different mouse models.