Boltzmann constant: Difference between revisions
From Bioblast
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
{{Keywords SI base units}} | {{Keywords SI base units}} | ||
{{Keywords: Concentration and pressure}} | |||
== References == | == References == | ||
Revision as of 12:24, 16 February 2020
Description
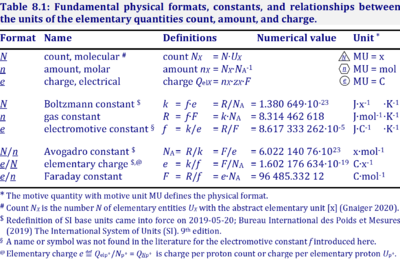
The Boltzmann constant, k, has the SI unit [J·K-1] (IUPAC), but more strictly the units for energy per particles per temperature is [J·x-1·K-1] (compare Gas constant).
Abbreviation: k [J·x-1·K-1]
Reference: Gibney 2017 Nature
Communicated by Gnaiger E 2018-10-18
Template:Keywords SI base units
- Bioblast links: Concentration and pressure - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Concentration
- » Volume
- » Activity
- » Concentration
- » Density
- » Mole
- » Molar mass
- Concentration
- Pressure
- Solubility = concentration/pressure
- General
- » Boltzmann constant
- » Energy
- » Force
- » Gas constant
- » Work
- General
- Related keyword lists
References
- Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - »Open Access pdf«
- Gnaiger E (2019) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Mitochondr Physiol Network 24.05. Oroboros MiPNet Publications, Innsbruck:112 pp. - »Bioblast link«
MitoPedia concepts:
Ergodynamics