Difference between revisions of "Ingram 2020 MitoFit Preprint Arch"
| Line 20: | Line 20: | ||
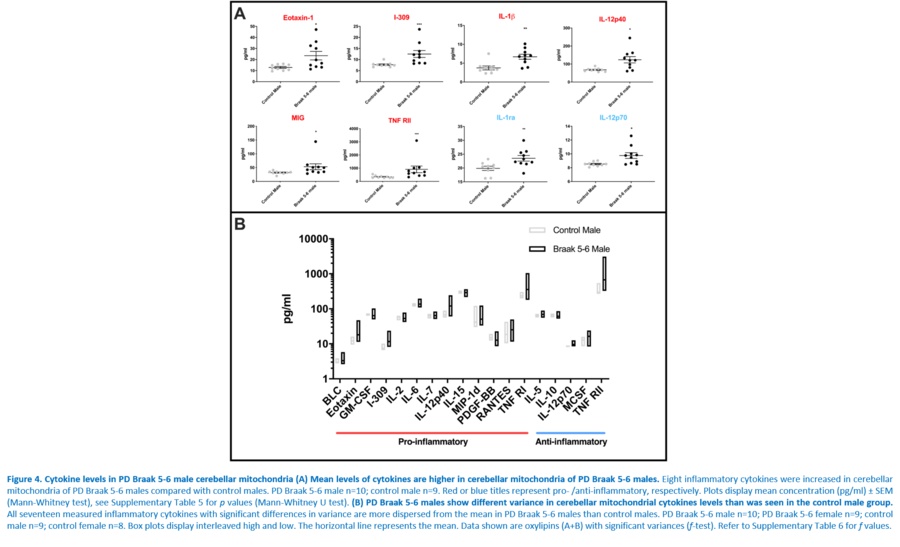
[[File:Chakrabarti Figure 4.png|900px]] | [[File:Chakrabarti Figure 4.png|900px]] | ||
== References == | == References == | ||
:::# | :::# Alafuzoff, I. et al. (2009) ‘Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium.’, Acta neuropathologica, 117(6), pp. 635–52. doi: 10.1007/s00401-009-0523-2. | ||
:::# | :::# Area-Gomez, E. et al. (2019) ‘Mitochondria, OxPhos, and neurodegeneration: Cells are not just running out of gas’, Journal of Clinical Investigation. American Society for Clinical Investigation. doi: 10.1172/JCI120848. | ||
:::# | :::# Balgoma, D. et al. (2013) ‘Quantitative metabolic profiling of lipid mediators’, Molecular Nutrition & Food Research, 57(8), pp. 1359–1377. doi: 10.1002/mnfr.201200840. | ||
:::# | :::# Blesa, J. et al. (2017) ‘Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification’, Experimental Neurology. Academic Press Inc., pp. 148–161. doi: 10.1016/j.expneurol.2017.10.002. | ||
:::# | :::# Bose, A. and Beal, M. F. (2016) ‘Mitochondrial dysfunction in Parkinson’s disease’, Journal of Neurochemistry, 139, pp. 216–231. doi: 10.1111/jnc.13731. | ||
:::# | :::# Caligiuri, S. P. B. et al. (2017) ‘Dietary modulation of oxylipins in cardiovascular disease and aging’, American Journal of Physiology-Heart and Circulatory Physiology, 313(5), pp. H903–H918. doi: 10.1152/ajpheart.00201.2017. | ||
:::# | :::# Caranci, G. et al. (2013) ‘Gender differences in Parkinson’s disease: focus on plasma α-synuclein.’, Journal of neural transmission (Vienna, Austria : 1996), 120(8), pp. 1209–15. doi: 10.1007/s00702-013-0972-6. | ||
:::# | :::# Chandra, G. et al. (2016) ‘Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease’, Journal of Biological Chemistry, 291(29), pp. 15267–15281. doi: 10.1074/jbc.M116.714824. | ||
:::# | :::# Chen, W. et al. (2019) ‘Gender differences in prevalence of LRRK2-associated Parkinson disease:A meta-analysis of observational studies.’, Neuroscience letters, p. 134609. doi: 10.1016/j.neulet.2019.134609. | ||
:::# | :::# Dewing, P. et al. (2006) ‘Direct regulation of adult brain function by the male-specific factor SRY’, Current Biology. Curr Biol, 16(4), pp. 415–420. doi: 10.1016/j.cub.2006.01.017. | ||
:::# | :::# Dexter, D. T. et al. (1989) ‘Basal Lipid Peroxidation in Substantia Nigra Is Increased in Parkinson’s Disease’, Journal of Neurochemistry. John Wiley & Sons, Ltd (10.1111), 52(2), pp. 381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. | ||
:::# | :::# Dirkx, M. F. et al. (2019) ‘Cerebral differences between dopamine-resistant and dopamine-responsive Parkinson’s tremor’, Brain, 142(10), pp. 3144–3157. doi: 10.1093/brain/awz261. | ||
:::# | :::# Eikelenboom, P. et al. (2010) ‘Neuroinflammation - An early event in both the history and pathogenesis of Alzheimer’s disease’, in Neurodegenerative Diseases. Neurodegener Dis, pp. 38–41. doi: 10.1159/000283480. | ||
:::# | :::# Erickson, M. A. et al. (2018) ‘Genetics and sex influence peripheral and central innate immune responses and blood-brain barrier integrity’, PLOS ONE. Edited by D. Janigro, 13(10), p. e0205769. doi: 10.1371/journal.pone.0205769. | ||
:::# | :::# Ferdouse, A. et al. (2019) ‘The Brain Oxylipin Profile Is Resistant to Modulation by Dietary n-6 and n-3 Polyunsaturated Fatty Acids in Male and Female Rats’, Lipids. John Wiley and Sons Inc., 54(1), pp. 67–80. doi: 10.1002/lipd.12122. | ||
:::# | :::# Fischer, R. et al. (2011) ‘A TNF receptor 2 selective agonist rescues human neurons from oxidative stress-induced cell death’, PLoS ONE. PLoS One, 6(11). doi: 10.1371/journal.pone.0027621. | ||
:::# | :::# De Franceschi, G. et al. (2017) ‘α-Synuclein structural features inhibit harmful polyunsaturated fatty acid oxidation, suggesting roles in neuroprotection’, Journal of Biological Chemistry, 292(17), pp. 6927–6937. doi: 10.1074/jbc.M116.765149. | ||
:::# | :::# Garwood, C. J. et al. (2010) ‘Anti-inflammatory impact of minocycline in a mouse model of tauopathy’, Frontiers in Psychiatry. Front Psychiatry, 1(OCT). doi: 10.3389/fpsyt.2010.00136. | ||
:::# | :::# Giuliani, N. et al. (2001) ‘Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes’, Experimental Gerontology, 36(3), pp. 547–557. doi: 10.1016/S0531-5565(00)00220-5. | ||
:::# | :::# Goodfellow, P. N. and Lovell-Badge, R. (1993) ‘SRY and Sex Determination in Mammals’, Annual Review of Genetics. Annual Reviews, 27(1), pp. 71–92. doi: 10.1146/annurev.ge.27.120193.000443. | ||
:::# | :::# Guzman-Martinez, L. et al. (2019) ‘Neuroinflammation as a Common Feature of Neurodegenerative Disorders’, Frontiers in Pharmacology, 10, p. 1008. doi: 10.3389/fphar.2019.01008. | ||
:::# | :::# Haque, M. E. et al. (2019) ‘Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease’, Movement Disorders, p. mds.27874. doi: 10.1002/mds.27874. | ||
:::# | :::# Hazeldine, J. et al. (2019) ‘Traumatic Injury and Exposure to Mitochondrial-Derived Damage Associated Molecular Patterns Suppresses Neutrophil Extracellular Trap Formation’, Frontiers in Immunology, 10, p. 685. doi: 10.3389/fimmu.2019.00685. | ||
:::# | :::# Kang, K. H. et al. (2013) ‘Protection of dopaminergic neurons by 5-lipoxygenase inhibitor’, Neuropharmacology. Elsevier Ltd, 73, pp. 380–387. doi: 10.1016/j.neuropharm.2013.06.014. | ||
:::# | :::# Kauppila, T. E. S., Kauppila, J. H. K. and Larsson, N. G. (2017) ‘Mammalian Mitochondria and Aging: An Update’, Cell Metabolism. Cell Press, pp. 57–71. doi: 10.1016/j.cmet.2016.09.017. | ||
:::# | :::# Kaut, O. et al. (2019) ‘5-methylcytosine and 5-hydroxymethylcytosine in brains of patients with multiple system atrophy and patients with Parkinson’s disease’, Journal of Chemical Neuroanatomy. Elsevier B.V., 96, pp. 41–48. doi: 10.1016/j.jchemneu.2018.12.005. | ||
:::# | :::# Krashia, P. et al. (2019) ‘Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease’, Nature Communications. Nature Publishing Group, 10(1). doi: 10.1038/s41467-019-11928-w. | ||
:::# | :::# Kumagai, T. et al. ‘Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease.’, Clinical neuropharmacology, 37(6), pp. 173–6. doi: 10.1097/WNF.0000000000000051. | ||
:::# | :::# Lee, H.-J. et al. (2010) ‘Brain arachidonic acid cascade enzymes are upregulated in a rat model of unilateral Parkinson disease.’, Neurochemical research, 35(4), pp. 613–9. doi: 10.1007/s11064-009-0106-6. | ||
:::# Lee, J. et al. (2019) ‘Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 116(33), pp. 16577–16582. doi: 10.1073/pnas.1900406116. | |||
:::# | :::# Leitner, G. R. et al. (2019) ‘Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders’, Expert Opinion on Therapeutic Targets, 23(10), pp. 865–882. doi: 10.1080/14728222.2019.1676416. | ||
:::# | :::# Liakh, I. et al. (2019) ‘Modern Methods of Sample Preparation for the Analysis of Oxylipins in Biological Samples.’, Molecules (Basel, Switzerland). Multidisciplinary Digital Publishing Institute (MDPI), 24(8). doi: 10.3390/molecules24081639. | ||
:::# Lucas, S. M., Rothwell, N. J. and Gibson, R. M. (2006) ‘The role of inflammation in CNS injury and disease’, British Journal of Pharmacology. Br J Pharmacol. doi: 10.1038/sj.bjp.0706400. | |||
:::# | :::# Marriott, I. and Huet-Hudson, Y. M. (2006) ‘Sexual dimorphism in innate immune responses to infectious organisms’, Immunologic Research, pp. 177–192. doi: 10.1385/IR:34:3:177. | ||
:::# | :::# Mattson, M. P. and Arumugam, T. V. (2018) ‘Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States’, Cell Metabolism. Cell Press, pp. 1176–1199. doi: 10.1016/j.cmet.2018.05.011. | ||
: | :::# McCarthy, M. M., Nugent, B. M. and Lenz, K. M. (2017) ‘Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain’, Nature Reviews Neuroscience. Nature Publishing Group, pp. 471–484. doi: 10.1038/nrn.2017.61. | ||
:::# | :::# McWilliams, T. G. et al. (2018) ‘Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand’, Cell Metabolism. Cell Press, 27(2), pp. 439-449.e5. doi: 10.1016/j.cmet.2017.12.008. | ||
:::# | :::# Mirdamadi, J. L. (2016) ‘Cerebellar Role in Parkinson’s Disease.’, Journal of neurophysiology, p. jn.01132.2015. doi: 10.1152/jn.01132.2015. | ||
:::# | :::# Mounsey, R. B. et al. (2015) ‘Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease’, Experimental Neurology, 273, pp. 36–44. doi: 10.1016/j.expneurol.2015.07.024. | ||
:::# | :::# Newcombe, E. A. et al. (2018) ‘Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease 11 Medical and Health Sciences 1109 Neurosciences 11 Medical and Health Sciences 1107 Immunology’, Journal of Neuroinflammation. BioMed Central Ltd. doi: 10.1186/s12974-018-1313-3. | ||
:::# | :::# Newman, L. E. and Shadel, G. S. (2018) ‘Pink1/Parkin link inflammation, mitochondrial stress, and neurodegeneration’, The Journal of cell biology. NLM (Medline), pp. 3327–3329. doi: 10.1083/jcb.201808118. | ||
:::# | :::# Pace, S., Sautebin, L. and Werz, O. (2017) ‘Sex-biased eicosanoid biology: Impact for sex differences in inflammation and consequences for pharmacotherapy’, Biochemical Pharmacology. Elsevier Inc., pp. 1–11. doi: 10.1016/j.bcp.2017.06.128. | ||
:::# | :::# Palmer, S. J. et al. (2010) ‘Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease’, Neuroscience, 166(4), pp. 1110–1118. doi: 10.1016/j.neuroscience.2010.01.012. | ||
:::# | :::# Picillo, M. et al. (2017) ‘The relevance of gender in Parkinson’s disease: a review’, Journal of Neurology, 264(8), pp. 1583–1607. doi: 10.1007/s00415-016-8384-9. | ||
:::# | :::# Pochard, C. et al. (2018) ‘Cyclooxygenase 2 is upregulated in the gastrointestinal tract in Parkinson’s disease’, Movement Disorders. John Wiley and Sons Inc., 33(3), pp. 493–494. doi: 10.1002/mds.27237. | ||
:::# | :::# Rajamani, A. et al. (2019) ‘Oxylipins in triglyceride-rich lipoproteins of dyslipidemic subjects promote endothelial inflammation following a high fat meal’, Scientific Reports, 9(1), p. 8655. doi: 10.1038/s41598-019-45005-5. | ||
:::# | :::# Ramsden, C. E. et al. (2016) ‘Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids’, Molecular Pain, 12, p. 174480691663638. doi: 10.1177/1744806916636386. | ||
:::# Refolo, V. and Stefanova, N. (2019) ‘Neuroinflammation and Glial Phenotypic Changes in Alpha-Synucleinopathies’, Frontiers in Cellular Neuroscience, 13, p. 263. doi: 10.3389/fncel.2019.00263. | |||
:::# | :::# Richardson, D. et al. (2007) ‘Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry.’, Analytical biochemistry, 360(2), pp. 216–26. doi: 10.1016/j.ab.2006.10.039. | ||
:::# | :::# Rieusset, J. (2018) ‘Mitochondria-associated membranes (MAMs): An emerging platform connecting energy and immune sensing to metabolic flexibility’, Biochemical and Biophysical Research Communications, 500(1), pp. 35–44. doi: 10.1016/j.bbrc.2017.06.097. | ||
:::# | :::# Scotland, R. S. et al. (2011) ‘Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice’, Blood, 118(22), pp. 5918–5927. doi: 10.1182/blood-2011-03-340281. | ||
:::# | :::# Seidel, K. et al. (2017) ‘Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies’, Annals of Neurology. John Wiley and Sons Inc., 81(6), pp. 898–903. doi: 10.1002/ana.24937. | ||
:::# | :::# Shephard, F. et al. (2013) ‘A mitochondrial location for haemoglobins-Dynamic distribution in ageing and Parkinson’s disease.’, Mitochondrion, 14(1), pp. 64–72. doi: 10.1016/j.mito.2013.12.001. | ||
:::# | :::# Shephard, F. et al. (2014) ‘A mitochondrial location for haemoglobins--dynamic distribution in ageing and Parkinson’s disease.’, Mitochondrion, 14(1), pp. 64–72. doi: 10.1016/j.mito.2013.12.001. | ||
:::# Siani, F. et al. (2017) ‘Influence of Estrogen Modulation on Glia Activation in a Murine Model of Parkinson’s Disease’, Frontiers in Neuroscience, 11, p. 306. doi: 10.3389/fnins.2017.00306. | |||
:::# | :::# Sigma-Aldrich (2013) ‘Technical bulletin: Bradford Reagent B6916’, pp. 3–8. | ||
:::# | :::# Sliter, D. A. et al. (2018a) ‘Parkin and PINK1 mitigate STING-induced inflammation’, Nature. doi: 10.1038/s41586-018-0448-9. | ||
:::# | :::# Sliter, D. A. et al. (2018b) ‘Parkin and PINK1 mitigate STING-induced inflammation’, Nature. doi: 10.1038/s41586-018-0448-9. | ||
:::# | :::# Smith, K. M. and Dahodwala, N. (2014) ‘Sex differences in Parkinson’s disease and other movement disorders.’, Experimental neurology, 259, pp. 44–56. doi: 10.1016/j.expneurol.2014.03.010. | ||
:::# | :::# Stöger, R., Scaife, Paula J, et al. (2017) ‘Elevated 5hmC levels characterize DNA of the cerebellum in Parkinson’s disease.’, NPJ Parkinson’s disease, 3, p. 6. doi: 10.1038/s41531-017-0007-3. | ||
:::# Stöger, R., Scaife, Paula J., et al. (2017) ‘Elevated 5hmC levels characterize DNA of the cerebellum in Parkinson’s disease’, npj Parkinson’s Disease. Nature Publishing Group, 3(1), p. 6. doi: 10.1038/s41531-017-0007-3. | |||
:::# Stojkovska, I., Wagner, B. M. and Morrison, B. E. (2015) ‘Parkinson’s disease and enhanced inflammatory response’, Experimental Biology and Medicine. SAGE Publications Inc., pp. 1387–1395. doi: 10.1177/1535370215576313. | |||
:::# | :::# Vivekanantham, S. et al. (2015) ‘Neuroinflammation in Parkinson’s disease: role in neurodegeneration and tissue repair’, International Journal of Neuroscience, 125(10), pp. 717–725. doi: 10.3109/00207454.2014.982795. | ||
:::# | :::# Weiduschat, N. et al. (2014) ‘Sex differences in cerebral energy metabolism in Parkinson’s disease: A phosphorus magnetic resonance spectroscopic imaging study.’, Parkinsonism & related disorders. doi: 10.1016/j.parkreldis.2014.02.003. | ||
:::# | :::# Wong, A. et al. (2014) ‘Simultaneous tissue profiling of eicosanoid and endocannabinoid lipid families in a rat model of osteoarthritis.’, Journal of lipid research, 55(9), pp. 1902–13. doi: 10.1194/jlr.M048694. | ||
:::# | :::# Wu, T. and Hallett, M. (2013) ‘The cerebellum in Parkinson’s disease.’, Brain : a journal of neurology, 136(Pt 3), pp. 696–709. doi: 10.1093/brain/aws360. | ||
:::# | :::# Yoo, H. S. et al. (2019) ‘Cerebellar connectivity in Parkinson’s disease with levodopa‐induced dyskinesia’, Annals of Clinical and Translational Neurology, p. acn3.50918. doi: 10.1002/acn3.50918. | ||
:::# | :::# Zhang, J. et al. (2015) ‘Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of intrinsic brain activity.’, Parkinsonism & related disorders, 21(1), pp. 23–30. doi: 10.1016/j.parkreldis.2014.10.017. | ||
== Preprints for [[Gentle Science]] == | == Preprints for [[Gentle Science]] == | ||
Revision as of 17:19, 29 April 2020
Ingram 2020 MitoFit Preprint Arch
| Ingram TL, Shephard F, Sarmad S, Ortori CA, Barrett DA and Chakrabart L (2020) Sex differences characterise inflammatory profiles of cerebellar mitochondria and are attenuated in Parkinson’s disease. MitoFit Preprint Arch doi:10.26124/mitofit:200002. |
»
Thomas L Ingram, Freya Shephard, Sarir Sarmad, Catherine A. Ortori, David A. Barrett and Lisa Chakrabarti. (2020) MitoFit Preprint Arch
Abstract: Version 1 (v1) 2020-04-23 doi:10.26124/mitofit:200002
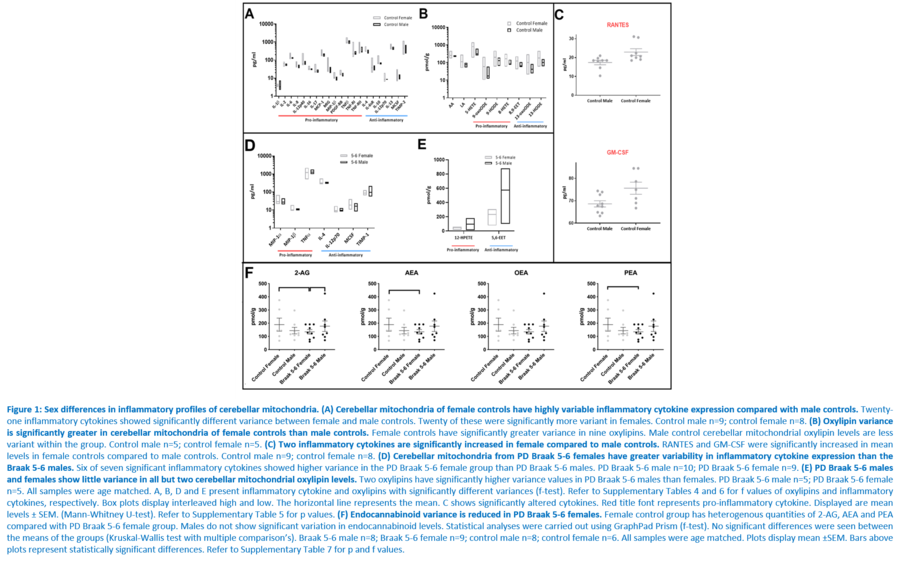
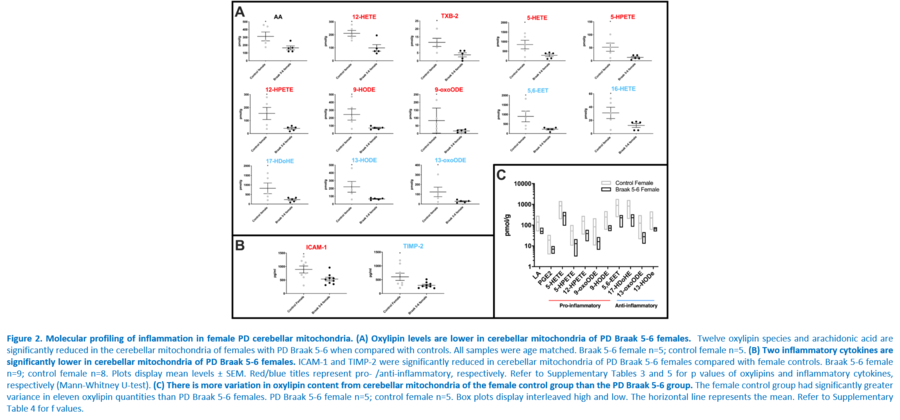
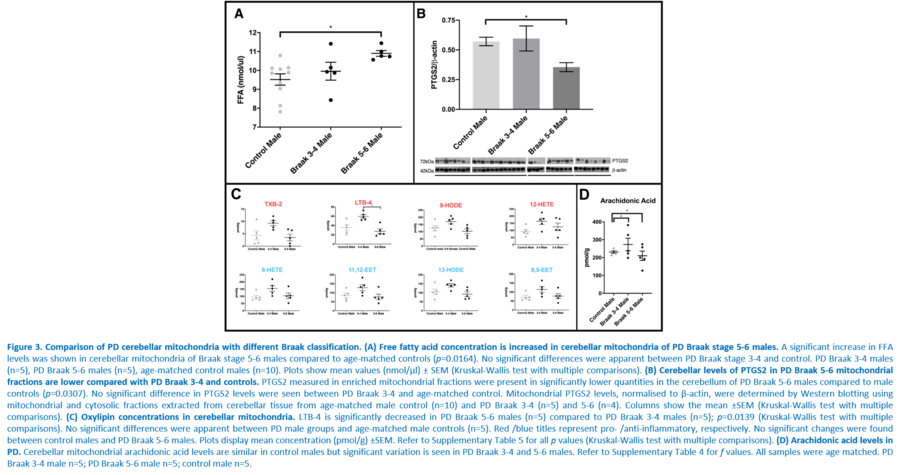
Response to inflammation is a key determinant in many diseases and their outcomes. Diseases that commonly affect older people are frequently associated with altered inflammatory processes. Neuroinflammation has been described in Parkinson’s disease (PD) brain and presents a potential therapeutic target. PD is characterised by the loss of dopaminergic neurons in the substantia nigra pars compacta and at the sub-cellular level, mitochondrial dysfunction is a key feature. However, there is evidence that a different region of the brain, the cerebellum, is involved in the pathophysiology of PD. We report relative levels of 40 pro- and anti-inflammatory cytokines measured in PD and control cerebellar mitochondria. These data were obtained by screening cytokine antibody arrays. In parallel, we present concentrations of 29 oxylipins and 4 endocannabinoids measured in mitochondrial fractions isolated from post-mortem PD cerebellum with age and sex matched controls. Our oxylipin and endocannabinoid data were acquired via quantitation by LC-ESI--MS/MS. The separate sample sets both show there are clearly different inflammatory profiles between the sexes in control samples. Sex specific profiles were not maintained in cerebellar mitochondria isolated from PD brains. One interpretation of our findings is that normally females have a wide-ranging inflammatory profile that can respond to or absorb the effects of increased levels of cytokines and oxylipins. These observations may have implications for other inflammatory diseases where the sexes are affected unequally in number or severity. • Keywords: Inflammation, Parkinson's disease, cerebellar mitochondria, sex differences • Bioblast editor: Iglesias-Gonzalez J
Figures
References
- Alafuzoff, I. et al. (2009) ‘Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium.’, Acta neuropathologica, 117(6), pp. 635–52. doi: 10.1007/s00401-009-0523-2.
- Area-Gomez, E. et al. (2019) ‘Mitochondria, OxPhos, and neurodegeneration: Cells are not just running out of gas’, Journal of Clinical Investigation. American Society for Clinical Investigation. doi: 10.1172/JCI120848.
- Balgoma, D. et al. (2013) ‘Quantitative metabolic profiling of lipid mediators’, Molecular Nutrition & Food Research, 57(8), pp. 1359–1377. doi: 10.1002/mnfr.201200840.
- Blesa, J. et al. (2017) ‘Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification’, Experimental Neurology. Academic Press Inc., pp. 148–161. doi: 10.1016/j.expneurol.2017.10.002.
- Bose, A. and Beal, M. F. (2016) ‘Mitochondrial dysfunction in Parkinson’s disease’, Journal of Neurochemistry, 139, pp. 216–231. doi: 10.1111/jnc.13731.
- Caligiuri, S. P. B. et al. (2017) ‘Dietary modulation of oxylipins in cardiovascular disease and aging’, American Journal of Physiology-Heart and Circulatory Physiology, 313(5), pp. H903–H918. doi: 10.1152/ajpheart.00201.2017.
- Caranci, G. et al. (2013) ‘Gender differences in Parkinson’s disease: focus on plasma α-synuclein.’, Journal of neural transmission (Vienna, Austria : 1996), 120(8), pp. 1209–15. doi: 10.1007/s00702-013-0972-6.
- Chandra, G. et al. (2016) ‘Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease’, Journal of Biological Chemistry, 291(29), pp. 15267–15281. doi: 10.1074/jbc.M116.714824.
- Chen, W. et al. (2019) ‘Gender differences in prevalence of LRRK2-associated Parkinson disease:A meta-analysis of observational studies.’, Neuroscience letters, p. 134609. doi: 10.1016/j.neulet.2019.134609.
- Dewing, P. et al. (2006) ‘Direct regulation of adult brain function by the male-specific factor SRY’, Current Biology. Curr Biol, 16(4), pp. 415–420. doi: 10.1016/j.cub.2006.01.017.
- Dexter, D. T. et al. (1989) ‘Basal Lipid Peroxidation in Substantia Nigra Is Increased in Parkinson’s Disease’, Journal of Neurochemistry. John Wiley & Sons, Ltd (10.1111), 52(2), pp. 381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x.
- Dirkx, M. F. et al. (2019) ‘Cerebral differences between dopamine-resistant and dopamine-responsive Parkinson’s tremor’, Brain, 142(10), pp. 3144–3157. doi: 10.1093/brain/awz261.
- Eikelenboom, P. et al. (2010) ‘Neuroinflammation - An early event in both the history and pathogenesis of Alzheimer’s disease’, in Neurodegenerative Diseases. Neurodegener Dis, pp. 38–41. doi: 10.1159/000283480.
- Erickson, M. A. et al. (2018) ‘Genetics and sex influence peripheral and central innate immune responses and blood-brain barrier integrity’, PLOS ONE. Edited by D. Janigro, 13(10), p. e0205769. doi: 10.1371/journal.pone.0205769.
- Ferdouse, A. et al. (2019) ‘The Brain Oxylipin Profile Is Resistant to Modulation by Dietary n-6 and n-3 Polyunsaturated Fatty Acids in Male and Female Rats’, Lipids. John Wiley and Sons Inc., 54(1), pp. 67–80. doi: 10.1002/lipd.12122.
- Fischer, R. et al. (2011) ‘A TNF receptor 2 selective agonist rescues human neurons from oxidative stress-induced cell death’, PLoS ONE. PLoS One, 6(11). doi: 10.1371/journal.pone.0027621.
- De Franceschi, G. et al. (2017) ‘α-Synuclein structural features inhibit harmful polyunsaturated fatty acid oxidation, suggesting roles in neuroprotection’, Journal of Biological Chemistry, 292(17), pp. 6927–6937. doi: 10.1074/jbc.M116.765149.
- Garwood, C. J. et al. (2010) ‘Anti-inflammatory impact of minocycline in a mouse model of tauopathy’, Frontiers in Psychiatry. Front Psychiatry, 1(OCT). doi: 10.3389/fpsyt.2010.00136.
- Giuliani, N. et al. (2001) ‘Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes’, Experimental Gerontology, 36(3), pp. 547–557. doi: 10.1016/S0531-5565(00)00220-5.
- Goodfellow, P. N. and Lovell-Badge, R. (1993) ‘SRY and Sex Determination in Mammals’, Annual Review of Genetics. Annual Reviews, 27(1), pp. 71–92. doi: 10.1146/annurev.ge.27.120193.000443.
- Guzman-Martinez, L. et al. (2019) ‘Neuroinflammation as a Common Feature of Neurodegenerative Disorders’, Frontiers in Pharmacology, 10, p. 1008. doi: 10.3389/fphar.2019.01008.
- Haque, M. E. et al. (2019) ‘Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease’, Movement Disorders, p. mds.27874. doi: 10.1002/mds.27874.
- Hazeldine, J. et al. (2019) ‘Traumatic Injury and Exposure to Mitochondrial-Derived Damage Associated Molecular Patterns Suppresses Neutrophil Extracellular Trap Formation’, Frontiers in Immunology, 10, p. 685. doi: 10.3389/fimmu.2019.00685.
- Kang, K. H. et al. (2013) ‘Protection of dopaminergic neurons by 5-lipoxygenase inhibitor’, Neuropharmacology. Elsevier Ltd, 73, pp. 380–387. doi: 10.1016/j.neuropharm.2013.06.014.
- Kauppila, T. E. S., Kauppila, J. H. K. and Larsson, N. G. (2017) ‘Mammalian Mitochondria and Aging: An Update’, Cell Metabolism. Cell Press, pp. 57–71. doi: 10.1016/j.cmet.2016.09.017.
- Kaut, O. et al. (2019) ‘5-methylcytosine and 5-hydroxymethylcytosine in brains of patients with multiple system atrophy and patients with Parkinson’s disease’, Journal of Chemical Neuroanatomy. Elsevier B.V., 96, pp. 41–48. doi: 10.1016/j.jchemneu.2018.12.005.
- Krashia, P. et al. (2019) ‘Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease’, Nature Communications. Nature Publishing Group, 10(1). doi: 10.1038/s41467-019-11928-w.
- Kumagai, T. et al. ‘Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease.’, Clinical neuropharmacology, 37(6), pp. 173–6. doi: 10.1097/WNF.0000000000000051.
- Lee, H.-J. et al. (2010) ‘Brain arachidonic acid cascade enzymes are upregulated in a rat model of unilateral Parkinson disease.’, Neurochemical research, 35(4), pp. 613–9. doi: 10.1007/s11064-009-0106-6.
- Lee, J. et al. (2019) ‘Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 116(33), pp. 16577–16582. doi: 10.1073/pnas.1900406116.
- Leitner, G. R. et al. (2019) ‘Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders’, Expert Opinion on Therapeutic Targets, 23(10), pp. 865–882. doi: 10.1080/14728222.2019.1676416.
- Liakh, I. et al. (2019) ‘Modern Methods of Sample Preparation for the Analysis of Oxylipins in Biological Samples.’, Molecules (Basel, Switzerland). Multidisciplinary Digital Publishing Institute (MDPI), 24(8). doi: 10.3390/molecules24081639.
- Lucas, S. M., Rothwell, N. J. and Gibson, R. M. (2006) ‘The role of inflammation in CNS injury and disease’, British Journal of Pharmacology. Br J Pharmacol. doi: 10.1038/sj.bjp.0706400.
- Marriott, I. and Huet-Hudson, Y. M. (2006) ‘Sexual dimorphism in innate immune responses to infectious organisms’, Immunologic Research, pp. 177–192. doi: 10.1385/IR:34:3:177.
- Mattson, M. P. and Arumugam, T. V. (2018) ‘Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States’, Cell Metabolism. Cell Press, pp. 1176–1199. doi: 10.1016/j.cmet.2018.05.011.
- McCarthy, M. M., Nugent, B. M. and Lenz, K. M. (2017) ‘Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain’, Nature Reviews Neuroscience. Nature Publishing Group, pp. 471–484. doi: 10.1038/nrn.2017.61.
- McWilliams, T. G. et al. (2018) ‘Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand’, Cell Metabolism. Cell Press, 27(2), pp. 439-449.e5. doi: 10.1016/j.cmet.2017.12.008.
- Mirdamadi, J. L. (2016) ‘Cerebellar Role in Parkinson’s Disease.’, Journal of neurophysiology, p. jn.01132.2015. doi: 10.1152/jn.01132.2015.
- Mounsey, R. B. et al. (2015) ‘Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease’, Experimental Neurology, 273, pp. 36–44. doi: 10.1016/j.expneurol.2015.07.024.
- Newcombe, E. A. et al. (2018) ‘Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease 11 Medical and Health Sciences 1109 Neurosciences 11 Medical and Health Sciences 1107 Immunology’, Journal of Neuroinflammation. BioMed Central Ltd. doi: 10.1186/s12974-018-1313-3.
- Newman, L. E. and Shadel, G. S. (2018) ‘Pink1/Parkin link inflammation, mitochondrial stress, and neurodegeneration’, The Journal of cell biology. NLM (Medline), pp. 3327–3329. doi: 10.1083/jcb.201808118.
- Pace, S., Sautebin, L. and Werz, O. (2017) ‘Sex-biased eicosanoid biology: Impact for sex differences in inflammation and consequences for pharmacotherapy’, Biochemical Pharmacology. Elsevier Inc., pp. 1–11. doi: 10.1016/j.bcp.2017.06.128.
- Palmer, S. J. et al. (2010) ‘Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease’, Neuroscience, 166(4), pp. 1110–1118. doi: 10.1016/j.neuroscience.2010.01.012.
- Picillo, M. et al. (2017) ‘The relevance of gender in Parkinson’s disease: a review’, Journal of Neurology, 264(8), pp. 1583–1607. doi: 10.1007/s00415-016-8384-9.
- Pochard, C. et al. (2018) ‘Cyclooxygenase 2 is upregulated in the gastrointestinal tract in Parkinson’s disease’, Movement Disorders. John Wiley and Sons Inc., 33(3), pp. 493–494. doi: 10.1002/mds.27237.
- Rajamani, A. et al. (2019) ‘Oxylipins in triglyceride-rich lipoproteins of dyslipidemic subjects promote endothelial inflammation following a high fat meal’, Scientific Reports, 9(1), p. 8655. doi: 10.1038/s41598-019-45005-5.
- Ramsden, C. E. et al. (2016) ‘Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids’, Molecular Pain, 12, p. 174480691663638. doi: 10.1177/1744806916636386.
- Refolo, V. and Stefanova, N. (2019) ‘Neuroinflammation and Glial Phenotypic Changes in Alpha-Synucleinopathies’, Frontiers in Cellular Neuroscience, 13, p. 263. doi: 10.3389/fncel.2019.00263.
- Richardson, D. et al. (2007) ‘Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry.’, Analytical biochemistry, 360(2), pp. 216–26. doi: 10.1016/j.ab.2006.10.039.
- Rieusset, J. (2018) ‘Mitochondria-associated membranes (MAMs): An emerging platform connecting energy and immune sensing to metabolic flexibility’, Biochemical and Biophysical Research Communications, 500(1), pp. 35–44. doi: 10.1016/j.bbrc.2017.06.097.
- Scotland, R. S. et al. (2011) ‘Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice’, Blood, 118(22), pp. 5918–5927. doi: 10.1182/blood-2011-03-340281.
- Seidel, K. et al. (2017) ‘Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies’, Annals of Neurology. John Wiley and Sons Inc., 81(6), pp. 898–903. doi: 10.1002/ana.24937.
- Shephard, F. et al. (2013) ‘A mitochondrial location for haemoglobins-Dynamic distribution in ageing and Parkinson’s disease.’, Mitochondrion, 14(1), pp. 64–72. doi: 10.1016/j.mito.2013.12.001.
- Shephard, F. et al. (2014) ‘A mitochondrial location for haemoglobins--dynamic distribution in ageing and Parkinson’s disease.’, Mitochondrion, 14(1), pp. 64–72. doi: 10.1016/j.mito.2013.12.001.
- Siani, F. et al. (2017) ‘Influence of Estrogen Modulation on Glia Activation in a Murine Model of Parkinson’s Disease’, Frontiers in Neuroscience, 11, p. 306. doi: 10.3389/fnins.2017.00306.
- Sigma-Aldrich (2013) ‘Technical bulletin: Bradford Reagent B6916’, pp. 3–8.
- Sliter, D. A. et al. (2018a) ‘Parkin and PINK1 mitigate STING-induced inflammation’, Nature. doi: 10.1038/s41586-018-0448-9.
- Sliter, D. A. et al. (2018b) ‘Parkin and PINK1 mitigate STING-induced inflammation’, Nature. doi: 10.1038/s41586-018-0448-9.
- Smith, K. M. and Dahodwala, N. (2014) ‘Sex differences in Parkinson’s disease and other movement disorders.’, Experimental neurology, 259, pp. 44–56. doi: 10.1016/j.expneurol.2014.03.010.
- Stöger, R., Scaife, Paula J, et al. (2017) ‘Elevated 5hmC levels characterize DNA of the cerebellum in Parkinson’s disease.’, NPJ Parkinson’s disease, 3, p. 6. doi: 10.1038/s41531-017-0007-3.
- Stöger, R., Scaife, Paula J., et al. (2017) ‘Elevated 5hmC levels characterize DNA of the cerebellum in Parkinson’s disease’, npj Parkinson’s Disease. Nature Publishing Group, 3(1), p. 6. doi: 10.1038/s41531-017-0007-3.
- Stojkovska, I., Wagner, B. M. and Morrison, B. E. (2015) ‘Parkinson’s disease and enhanced inflammatory response’, Experimental Biology and Medicine. SAGE Publications Inc., pp. 1387–1395. doi: 10.1177/1535370215576313.
- Vivekanantham, S. et al. (2015) ‘Neuroinflammation in Parkinson’s disease: role in neurodegeneration and tissue repair’, International Journal of Neuroscience, 125(10), pp. 717–725. doi: 10.3109/00207454.2014.982795.
- Weiduschat, N. et al. (2014) ‘Sex differences in cerebral energy metabolism in Parkinson’s disease: A phosphorus magnetic resonance spectroscopic imaging study.’, Parkinsonism & related disorders. doi: 10.1016/j.parkreldis.2014.02.003.
- Wong, A. et al. (2014) ‘Simultaneous tissue profiling of eicosanoid and endocannabinoid lipid families in a rat model of osteoarthritis.’, Journal of lipid research, 55(9), pp. 1902–13. doi: 10.1194/jlr.M048694.
- Wu, T. and Hallett, M. (2013) ‘The cerebellum in Parkinson’s disease.’, Brain : a journal of neurology, 136(Pt 3), pp. 696–709. doi: 10.1093/brain/aws360.
- Yoo, H. S. et al. (2019) ‘Cerebellar connectivity in Parkinson’s disease with levodopa‐induced dyskinesia’, Annals of Clinical and Translational Neurology, p. acn3.50918. doi: 10.1002/acn3.50918.
- Zhang, J. et al. (2015) ‘Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of intrinsic brain activity.’, Parkinsonism & related disorders, 21(1), pp. 23–30. doi: 10.1016/j.parkreldis.2014.10.017.
Preprints for Gentle Science
» MitoFit Preprints - the Open Access preprint server for mitochondrial physiology and bioenergetics
Labels: MiParea: mt-Medicine
Pathology: Parkinson's
Organism: Human