Pavlovic 2017 MITOEAGLE Obergurgl
| Establishing cellular model for studying mechanisms of muscle insulin resistance: towards understanding the role of mitochondria. |
Link: MitoEAGLE
Pavlovic K, Krako Jakovljevic N, Jovanovic M, Isakovic A, Markovic I, Lalic NM (2017)
Event: MitoEAGLE Obergurgl 2017
The activity of insulin affects the majority of cells in human body. The insulin signalling cascade has been widely studied in different cellular models, shedding light on its physiological and pathophysiological role. The latter was mostly relevant for understanding all types of Diabetes mellitus and their treatment solutions. Impairment of mitochondrial function has been linked to insulin resistance (inability of tissues to respond to insulin) [1]. There is still an issue in understanding the precise role of mitochondria in development of insulin resistance.
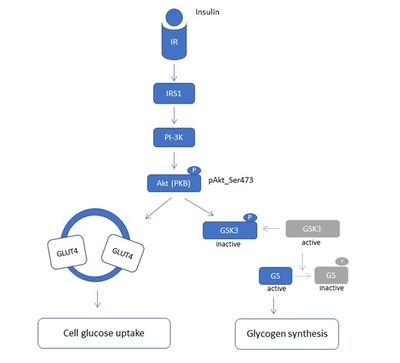
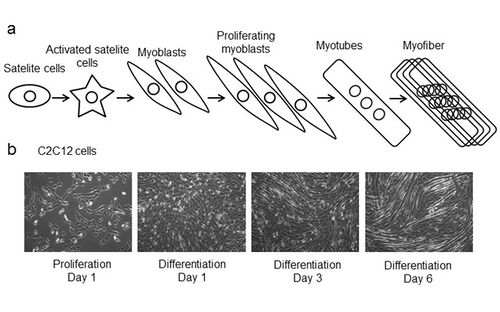
We established an in vitro model for studying cellular mechanisms of muscle insulin resistance (IR) in C2C12 myotubes. In contrast to other published cell models of insulin resistance in C2C12 cells, induced mostly by palmitate exposure [2], we exposed differentiated C2C12 myotubes to chronic insulin and observed lower insulin sensitivity. We followed the alterations of insulin sensitivity at the level of insulin signalling, by following the level of phosphorylation of protein kinase B (PKB or Akt) on serine 473 (pAkt-S473) by western blot. Akt is the key component of the insulin pathway linking insulin receptor (IR) and its substrates (IRSs) to the metabolic actions of insulin through the phosphoinositide 3-kinase (PI3-K) (Figure 1). We have characterized our in vitro insulin resistance model in terms of different insulin concentrations used, as well as chronic vs acute insulin treatment in different stages of C2C12 differentiation: from proliferative myoblasts to differentiated myotubes (Figure 2). This in vitro model might be highly relevant for better understanding of cellular and molecular mechanisms of IR in diabetic patients with hyperinsulinemia.
We have preliminary data on oxidative stress and mitochondrial morphology in this in vitro IR model, implying to the important role of mitochondria during insulin resistance. Considering its simplicity, this model will be easily applicable for conducting mitochondrial physiology studies, especially high-resolution respirometry that is of our great interest. We think that optimizing and applying substrate-uncoupler-inhibitor titration (SUIT) protocol [3] for detailed evaluation of mitochondrial respiration in this cell model will allow us to directly assess mitochondrial physiology of the insulin resistant state. This would also ideally fit with the main MitoEAGLE objective to improve our knowledge on mitochondrial function in health and disease [4]. Applying further diverse experimental conditions to this model will allow us to question the EAGLE (Evolution, Age, Gender, Lifestyle and Environment) effects on insulin resistance.
• Bioblast editor: Kandolf G
• O2k-Network Lab: RS Belgrade Lalic NM
Labels: MiParea: Respiration, mt-Medicine, mt-Awareness Pathology: Diabetes
Organism: Mouse Tissue;cell: Skeletal muscle
HRR: Oxygraph-2k Event: A1, Oral
Affiliations
- Pavlović K(1,2), Krako Jakovljević N(1), Jovanović M(3), Isaković A(3), Marković I(3), Lalić NM(1)
- Clinic Endocrinol, Diabetes Metabolic Diseases, Fac Medicine
- Inst Physiol Biochem, Fac Biol
- Inst Medical Clinical Biochem, Fac Medicine
- Univ Belgrade, Serbia. - [email protected]
Figures
Figure 1. Diagram of PI3K/Akt insulin signalling pathway. This pathway regulates the metabolic effects of insulin. IR – Insulin receptor, IRS1 – Insulin receptor substrate 1, PI-3K – Phosphoinositide 3 kinase, Akt (PKB) – Protein kinase B, GLUT4 – glucose transporter, GSK3 – Glycogen synthase kinase 3, GS – Glycogen synthase
Figure 2. (a) Scheme of satellite cell myogenesis: satellite cells (precursors to skeletal muscle cells) can be activated by muscle damage; activated satellite cells divide to produce myoblasts that further proliferate (proliferating myoblasts); cell to cell contact induce myoblast to differentiate and fuse into myotubes (multi-nucleated cells) which then mature into myofibers. (b) C2C12 cells, the mouse fast-fusing myoblasts, proliferate under standard cell culturing condition; when reach confluence C2C12 myoblast start differentiation into the myotubes that is enhanced by addition of 2% horse serum. The highest percentage of differentiated C2C12 myotubes was observed at differentiation day 6.
References and support
- Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307:384-87.
- Chavez JA, Summers SA (2003) Characterizing the effects of saturated fatty acids on insulin signalling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophysics 419:101-9.
- MitoPedia:_SUIT - Substrate-uncoupler-inhibitor protocols
- MitoEAGLE
Selected mentor: Pablo Miguel Garcia-Roves