Nehlin 2018 MiP2018
| Aging biomarkers in multimorbidity patients. Nehlin_Presentation |
Link: MiP2018
Nehlin JO, Tavenier J, Andersen O (2018)
Event: MiP2018
We wish to establish clinically-relevant aging biomarkers that can be associated with frailty level and disease state of chronically-ill, multimorbidity elderly patients that will contribute to create an estimate of their current physiological age, serving as a base for personalized treatment interventions.
Gradual accumulation of dysfunctional cells known as senescent cells has been correlated with age-related pathologies in many different types of tissues and organs, and their origin can be explained by various types of damage [1-3]. Cellular senescence is characterized by a series of morphological and physiological changes that ultimately lead to an irreversible state of proliferative arrest and resistance to apoptosis. A major consequence of senescence is the acquisition of a senescence-associated secretory phenotype (SASP) that increases the risk of cancer, inhibits angiogenesis, causes a pro-inflammatory state and alters the normal functioning of tissues, contributing to age-associated pathologies [2,4]. Interestingly, the composition of the SASP appears to vary according to the type of mechanism that induced the senescent state [5-7]. Mitochondria dysfunction can lead to senescence and is characterized by a distinct SASP profile [5,8]. The use of biomarkers of aging can help to reveal the true biological age of an individual [9-12], influenced by epigenetics and resilience-promoting factors [13,14], and could help predict health outcomes in multimorbidity patients. Several biomarker profiles are being analyzed.
• Bioblast editor: Plangger M
Labels: Pathology: Aging;senescence
Affiliations
- Clinical Research Center, Copenhagen Univ Hospital-Hvidovre, Denmark. - [email protected]
Support
This project is supported by regional funds from the Capital Region, the Clinical Research Center at Amager and Hvidovre hospitals, the Sofus Carl Emil Friis and Hustru Olga Doris Friis' scholarship and the Toyota Fund.
Figures
Figure 1. Design of the FAM-CPH cohort. In dark grey, the FAM-group consists of acutely ill patients aged 65 and over (n=128). In light grey, the Control group consists of citizen matched 1:1 on age, sex and municipality with patients from the FAM-group, but with no recent acute hospital admission (n=54). In italics and arrows, the aspects of biological aging that we will investigate: resilience as the development in aging markers between acute illness and baseline, the biological age as the difference in aging markers between the groups at baseline, the rate of aging as the development in aging markers between baseline and the 1-year follow-up.
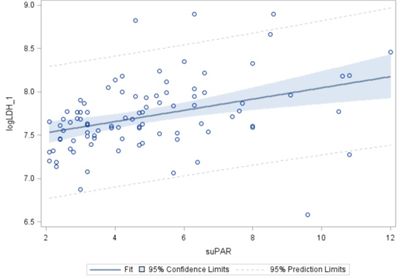
Figure 2. Example of biomarker analysis in acutely ill elderly patients
Plasma LDH and suPAR levels at admission, n = 91 – significant: p = 0.0003. The tissue-breakdown marker LDH (lactate dehydrogenase 1) [15] and the mortality marker suPAR (soluble urokinase-type plasminogen activator receptor) [16] are predictors of frailty and mortality.
References
- Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21:1424-35.
- Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM (2017) Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 16:718-35.
- Muñoz-Espin D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15:482-96.
- Kirkland JL, Tchkonia T (2017) Cellular senescence: A translational perspective. EBioMedicine 21:21-28.
- Wiley CD, Velarde MC, Lecot P, Gerencser AA, Verdin E, Campisi J (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23:303-14.
- Wiley CD, Campisi J (2016) From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab 23:1013-21.
- Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X, Unger MA, Vijg J, Melov S, Campisi J (2017) Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16:1043-50.
- Khrapko K, Vijg J (2009) Mitochondrial DNA mutations and aging: devils in the details. Trends Genet 25:91-98.
- Nehlin JO (2016) Biomarkers of replicative senescence revisited. Cellular ageing and replicative senescence. Healthy ageing and longevity 4:203-39.
- Sebastiani P, Thyagarajan B, Sun F, Schupf N, Newman AB, Montano M, Perls TT (2017) Biomarker signatures of ageing. Aging Cell 16:329-38.
- Bürkle A, Moreno-Villanueva M, Bernhard J, Blasco M, Zondag G, Hoeijmakers JH, Toussaint O, Grubeck-Loebenstein B, Mocchegiani E, Collino S, Gonos ES, Sikora E, Gradinaru D, Dollé M, Salmon M, Kristensen P, Griffiths HR, Libert C, Grune T, Breusing N, Simm A, Franceschi C, Capri M, Talbot D, Caiafa P, Friguet B, Slagboom PE, Hervonen A, Hurme M, Aspinall R (2015) MARK-AGE biomarkers of ageing. Mech Ageing Dev 151:2-12.
- Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M, Salvioli S (2018) The Continuum of ageing and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 5:61.
- Song Z, von Figura G, Liu Y, Kraus JM, Torrice C, Dillon P, Rudolph-Watabe M, Ju Z, Kestler HA, Sanoff H, Rudolph KL (2010) Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell 9:607-15.
- Fontana L, Addante F, Copetti M, Paroni G, Fontana A, Sancarlo D, Pellegrini F, Ferrucci L, Pilotto A (2013) Identification of a metabolic signature for multidimensional impairment and mortality risk in hospitalized older patients. Aging Cell 12:459–66.
- Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, Ortolano S, Pani G, Athanasopoulou S, Gonos ES, Schosserer M, Grillari J, Peterson P, Tuna BG, Dogan S, Meyer A, van Os R, Trendelenburg AU (2018) Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. pii:S1568-1637(18)30093-X.
- Rasmussen LJ, Ladelund S, Haupt TH, Ellekilde G, Poulsen JH, Iversen K, Eugen-Olsen J, Andersen O (2016) Soluble urokinase plasminogen activator receptor (suPAR) in acute care: a strong marker of disease presence and severity, readmission and mortality. Emerg Med J 33:769-75.