Crisostomo 2017 MITOEAGLE Obergurgl
| Glycerol metabolism in testicular cells – a mitochondrial tale that may control male reproductive potential. |
Link: MitoEAGLE
Crisostomo L, Sousa M, Barros A, Alves MG, Oliveira PF (2017)
Event: MitoEAGLE Obergurgl 2017
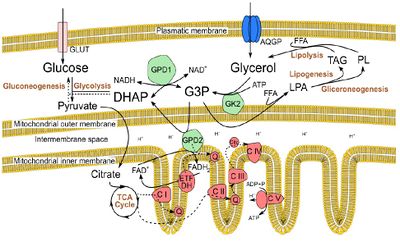
The worldwide increasing trend in the prevalence of type II diabetes mellitus (T2DM), obesity and reproductive dysfunctions have arisen the hypothesis that the increase in metabolic disorders is linked to lower birth rates [1]. However, the biochemical mechanisms underlying this association are far from being fully understood. Herein, we studied the hypothesis that glycerol, a by-product of triacylglycerols (Figure 1), may play a role in this association. When this polyol is present in testicular environment at high concentrations, it leads to an arrestment of spermatogenesis, by disrupting the integrity of the Sertoli cell/blood-testis barrier [2]. The testicular Sertoli cells (SCs) are key somatic cellular components of the testicular environment, providing structural and nutritional support to developing germ cells [3]. In fact, SC metabolism is essential for spermatogenesis and, hence, for male fertility [3] and it is known that these cells express aquaglyceroporins, being capable of importing glycerol [4].
In this work, we proposed to study the potential role of glycerol in Sertoli cell metabolism. We established primary human SC cultures, obtained from 6 biopsies, from men with conserved spermatogenesis subjected to testicular biopsy for spermatozoa retrieval, due to anajeculation or previous vasectomy. hSCs were then grown during 24 hours in control conditions (0 μM glycerol) or exposed to different glycerol concentrations (Infraphysiological - 0.1 μM; physiological – 10 μM; and supraphysiological - 1000 μM). We then evaluated glycerol cytotoxicity (by LDH assay) and how it affected cell viability (by MTT assay). In addition, we studied mitochondrial membrane potential (JC1 assay), and the protein expression levels (by Western Blot) of the electron transfer chain (ETC) complexes on the hSCs of all groups.
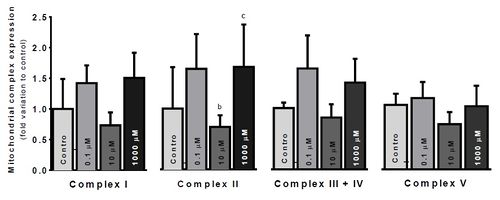
Our results showed a significant decrease in cytotoxicity under infraphysiological glycerol concentrations (P<0.05). However, no significant effects were observed on cell viability nor on mitochondrial membrane potential. The most significant differences were observed in ETC complexes protein expression (Figure 2), although they were only observed in complex II. Complex II expression was significantly depressed in the physiological group, comparing to infraphysiological (p<0.05) and supraphysiological groups (p<0.05).
Glycerol concentration significantly shifted ETC complexes protein expression. Overexpression of complex II under supraphysiological glycerol concentrations might be explained by an increased GPD2 activity, to metabolize the glycerol excess through the G3P shuttle (Figure 1). Complex I downregulation at physiological glycerol concentration group may reflect a balanced, ideal state between metabolic pathways linked by the intermediary metabolism. Further studies will be needed to unveil those mechanisms exposed by these preliminary results.
In conclusion, a fat-rich diet, associated with increased glycerol availability in the body, may induce significant metabolic changes in mitochondrial physiology and function of SC, which may result in severe alterations in spermatogenesis.
• Bioblast editor: Kandolf G
Labels:
Organism: Human
Tissue;cell: Genital
Enzyme: Complex I, Complex II;succinate dehydrogenase, Complex III, Complex IV;cytochrome c oxidase, Complex V;ATP synthase
Event: A1, Oral, Poster
Affiliations
- Crisóstomo L(1,2,3), Sousa M(1,4), Barros A(2,3,4), Alves MG(1), Oliveira PF(1,2,3)
- Dept Microscopy, Lab Cell Biol, Unit Multidisciplinary Research Biomedicine (UMIB), Inst Biomedical Sciences Abel Salazar (ICBAS), Univ of Porto
- Dept Genetics, Fac Medicine
- i3S – Inst Innovation Health Research, Univ Porto
- Centre Reproductive Genetics Prof Alberto Barros
- Porto, Portugal.- [email protected]
Figures
Figure 1. Glycerol metabolism, in Sertoli cells, in the context of intermediary metabolism. Glycerol is imported in Sertoli cells by aquaglyceroporins (AQGPs) or it is obtained as a result from lipolysis. After phosphorylation by glycerol kinase 2 (GK2), glycerol can enter the glycerol-3-P (G3P) shuttle or it can be conjugated with a free fatty acid (FFA) to form lysophosphatidic acid (LPA), entering lipogenesis or gliceroneogenesis. The G3P shuttle also allows glycerol to be directed towards glycolysis or gluconeogenesis, and potentially provides electrons for the electron transfer chain (ETC), via FAD+, in a reaction catalysed by the glycerol phosphate dehydrogenase 2 (GPD2), a mitocondrial enzyme.
Figure 2. Electron Transfer Chain (ETC) complexes relative protein expression, per treatment condition. Results are expressed as the average fold variation to control (No glycerol) ± SEM. Values were tested by one-way ANOVA with LSD multiple comparisons test, using GraphPad Prism v6. Results were accounted significant when p<0.05. Significant differences: a – relative to Control; b – relative to 0.1μM glycerol; c – relative to 10μM glycerol; d – relative to 1000μM glycerol
References
- Crisóstomo L, Sousa M, Alves MG, Oliveira PF (2017) The burden of metabolic diseases on male reproductive health. Int J Diabetol & Vascular Disease Research 5:1-2.
- Wiebe JP, Kowalik A, Gallardi RL, Egeler O, Clubb BH (2000) Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl 21:625-35.
- Oliveira PF, Sousa M, Silva BM, Monteiro MP, Alves MG (2017) Obesity, energy balance and spermatogenesis. Reproduction 153:R173-85
- Jesus TT, Bernardino RL, Martins AD, Sá R, Sousa M, Alves MG, Oliveira PF (2014) Aquaporin-9 is expressed in rat Sertoli cells and interacts with the cystic fibrosis transmembrane conductance regulator. IUBMB Life 66:639-44.
Acknowledgement and support
This work was supported by the Portuguese Foundation for Science and Technology: M.G. Alves (IFCT2015 and PTDC/BIM-MET/4712/2014); P.F. Oliveira (IFCT2015 and PTDC/BBB-BQB/1368/2014); UMIB (Pest-OE/SAU/UI0215/2014); co-funded by FEDER funds through the POCI/COMPETE 2020; and by the Portuguese Society of Diabetology (SPD) through the “Nuno Castel-Branco” research grant.
- Selected Mentor: Prof. Dr. Rui Miguel Pinheiro Vitorino, PhD.