| Baglivo E, Cardoso LHD, Cecatto C, Gnaiger E (2022) Statistical analysis of instrumental reproducibility as internal quality control in high-resolution respirometry. Bioenerg Commun 2022.8. https://doi.org/10.26124/bec:2022-0008 |
» Bioenerg Commun 2022.08. ![]()
published online 2022-08-04 ![]()
Baglivo Eleonora, Cardoso Luiza HD, Cecatto Cristiane, Gnaiger Erich (2022) Bioenerg Commun
Abstract: ![]() https://doi.org/10.26124/bec:2022-0008
https://doi.org/10.26124/bec:2022-0008
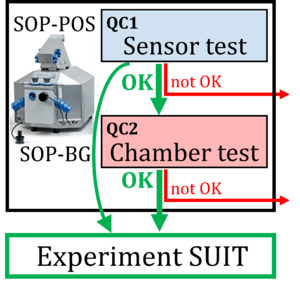
Evaluation of instrumental reproducibility is a primary component of quality control to quantify the precision and limit of detection of analytical procedures. A pre-analytical instrumental standard operating procedure (SOP) is implemented in high-resolution respirometry consisting of: (1) a daily SOP-POS for air calibration of the polarographic oxygen sensor (POS) in terms of oxygen concentration cO2 [µM]. This is part of the sensor test to evaluate POS performance; (2) a monthly SOP-BG starting with the SOP-POS followed by the chamber test quantifying the instrumental O2 background. The chamber test focuses on the slope dcO2/dt [pmol∙s−1∙mL−1] to determine O2 consumption by the POS and O2 backdiffusion into the chamber as a function of cO2 in the absence of sample. Finally, zero O2 calibration completes the sensor test.
We applied this SOP in a 3-year study using 48 Oroboros O2k chambers. Stability of air and zero O2 calibration signals was monitored throughout intervals of up to 8 months without sensor service. Maximum drift over 1 to 3 days was 0.06 pmol∙s−1∙mL−1, without persistence over time since drift was <0.004 pmol∙s−1∙mL−1 for time intervals of one month, corresponding to a drift per day of 0.2 % of the signal at air saturation. Instrumental O2 background -dcO2/dt was stable within ±1 pmol∙s−1∙mL−1 when measured at monthly intervals. These results confirm the instrumental limit of detection of volume-specific O2 flux at ±1 pmol∙s−1∙mL−1. The instrumental SOP applied in the present study contributes to the generally applicable internal quality control management ensuring the unique reproducibility in high-resolution respirometry.
• Keywords: high-resolution respirometry HRR; polarographic oxygen sensor POS; air calibration; instrumental background; reproducibility; limit of detection; internal quality control IQC; standard operating procedure SOP
• Bioblast editor: Tindle-Solomon L
• O2k-Network Lab: AT Innsbruck Oroboros
ORCID:![]() Baglivo Eleonora
Baglivo Eleonora ![]() Cardoso Luiza HD,
Cardoso Luiza HD, ![]() Cecatto Cristiane,
Cecatto Cristiane, ![]() Gnaiger Erich
Gnaiger Erich
Preprint
Support
- This work was part of the Oroboros NextGen-O2k project, with funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement nº 859770.
Labels: MiParea: Respiration, Instruments;methods
Tissue;cell: HEK
HRR: Oxygraph-2k, TIP2k, O2k-Protocol
BEC, SUIT-001 O2 ce-pce D003